
Don’t let a proofreading or translation mistake fence you off new markets.
Legal requirements to import cosmetics. Here’s all you need to know.

Photo by Element5 Digital on Unsplash
BBcream, lip gloss, blush, foundation, colorful shades and glitter. It could be a Power Puff Girls recipe, but it’s the products of an expanding industry. And the incorrect labeling of these cosmetics could derail your shipment at the port of entry to the US. Or any other country.
A PROFITABLE INDUSTRY
First things first, “cosmetics” is not an all encompassing entity. There are categories in the beauty industry. Five to be more precise: haircare, skincare, fragrance, make up, and hygiene. For a while now, since 2018, skin care seems to have taken over as the most profitable product category, with a market value of over 21billion.
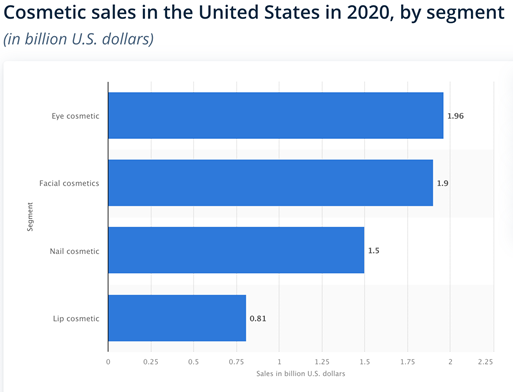
Within the cosmetics category, at least in the U.S, eye cosmetics is the most profitable segment. It aggregates approximately 1.96 billion USD generated from sales in 2020. Mascara takes the lead in this glamorous race, though eye liners, eye shadows, eyebrow makeup and eye combos don’t lag behind.
Who is winning the world beauty pageant? Asia Pacific is the industry leader. It accounts for 41% of the global cosmetic market, followed by the United States at 24%.
And where are those sales reported? In 2019, 14% of that revenue was made from online channels. And today, in some cases, revenues are twice their sales before the outbreak. So staying at home is not a deterrent for keeping a #wokeuplikethis look. And it’s not a women’s thing, men in the US and UK are shopping more frequently and consciously every day.
WHAT IS “COSMETICS”
One same product may be a cosmetic product in one region, and a quasi drug in another.
In the US the two most important cosmetic related laws are the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA). They define cosmetics by their intended use, as “articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or otherwise applied to the human body…for cleansing, beautifying, promoting attractiveness, or altering the appearance”. Except for soap.
The EU on the other hand, defines it as any substance or mixture intended to be placed in contact with the external parts of the human body. As long as the purpose is that of cleaning, perfuming, changing their appearance, keeping them in good condition or correcting body odours. (EU Regulation 1223/2009, Article 2.1.a)(EU Regulation 1223/2009, Article 2.1.a)
It seems subtle, but tiny variations have different legal implications. And so it goes for other countries around the world.
LEGAL REQUIREMENTS TO IMPORT COSMETICS
You don’t need FDA approval to go on the market, but there is a published cosmetic labeling guide. And the FDA can (and does) pursue enforcement action against products that don’t comply.
For example, the FD&C Act prohibits the marketing of adulterated or misbranded cosmetics in interstate commerce.
“Adulteration” could refer to violations involving ingredients, contaminants, processing, packaging, or shipping and handling.
And “Misbranding” to violations involving improperly labeled or deceptively packaged products. The container should not be misleading in any way, and all precautions must be explained.
If this is not the case, legal action can be pursued and the product could be removed from the market. A costly mistake for something easily avoidable.
WHAT MUST BE INCLUDED AND TRANSLATED
Here is a list of FDA cosmetic labeling requirements
- The common name of the product must be displayed prominently. Example “Moisturizing cream”
- So should its weight
- All the ingredients used (except flavors and fragrances) must appear on its label in order of prominence.
- Name and address of manufacturer or distributor should be included
- Warning statements if applicable
- Ingredients should be written according to their common name, for better public understanding.
And all this should be done in a predefined font size.
If you were looking more towards the east, here are the 10 must haves in labelling products in the EU.
Are you being even more expeditive? You can find out who oversees cosmetic claims, safety and ingredient compliance internationally. Here’s a small video on the subject.
Wherever you choose to sprinkle your cosmetic magic, at STILLMAN we can help. We take care there is a proper labelling translation. And that these are according to the regulations in each country so you don’t lose your touch.
